Search
- Page Path
- HOME > Search
- Diabetes, obesity and metabolism
- Hypothalamic AMP-Activated Protein Kinase as a Whole-Body Energy Sensor and Regulator
- Se Hee Min, Do Kyeong Song, Chan Hee Lee, Eun Roh, Min-Seon Kim
- Endocrinol Metab. 2024;39(1):1-11. Published online February 14, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1922
- 1,885 View
- 74 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
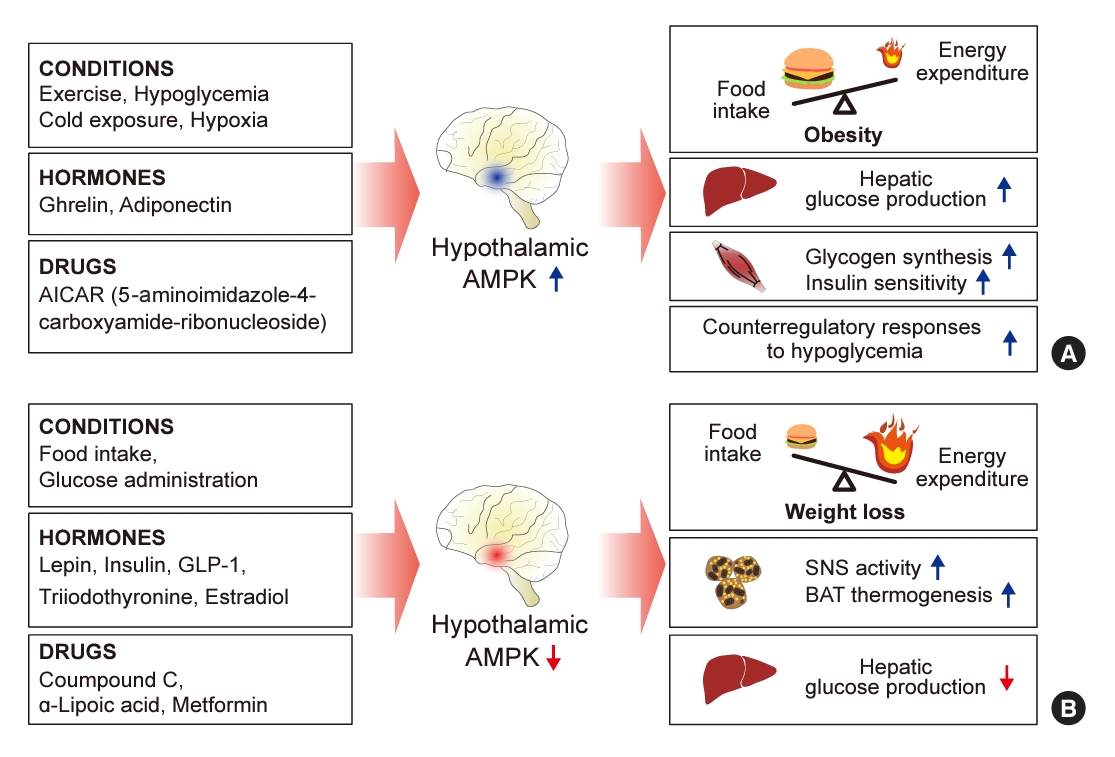
ePub - 5´-Adenosine monophosphate (AMP)-activated protein kinase (AMPK), a cellular energy sensor, is an essential enzyme that helps cells maintain stable energy levels during metabolic stress. The hypothalamus is pivotal in regulating energy balance within the body. Certain neurons in the hypothalamus are sensitive to fluctuations in food availability and energy stores, triggering adaptive responses to preserve systemic energy equilibrium. AMPK, expressed in these hypothalamic neurons, is instrumental in these regulatory processes. Hypothalamic AMPK activity is modulated by key metabolic hormones. Anorexigenic hormones, including leptin, insulin, and glucagon-like peptide 1, suppress hypothalamic AMPK activity, whereas the hunger hormone ghrelin activates it. These hormonal influences on hypothalamic AMPK activity are central to their roles in controlling food consumption and energy expenditure. Additionally, hypothalamic AMPK activity responds to variations in glucose concentrations. It becomes active during hypoglycemia but is deactivated when glucose is introduced directly into the hypothalamus. These shifts in AMPK activity within hypothalamic neurons are critical for maintaining glucose balance. Considering the vital function of hypothalamic AMPK in the regulation of overall energy and glucose balance, developing chemical agents that target the hypothalamus to modulate AMPK activity presents a promising therapeutic approach for metabolic conditions such as obesity and type 2 diabetes mellitus.

- Diabetes, Obesity and Metabolism
- Incretin and Pancreatic β-Cell Function in Patients with Type 2 Diabetes
- Chang Ho Ahn, Tae Jung Oh, Se Hee Min, Young Min Cho
- Endocrinol Metab. 2023;38(1):1-9. Published online February 13, 2023
- DOI: https://doi.org/10.3803/EnM.2023.103
- 3,333 View
- 362 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
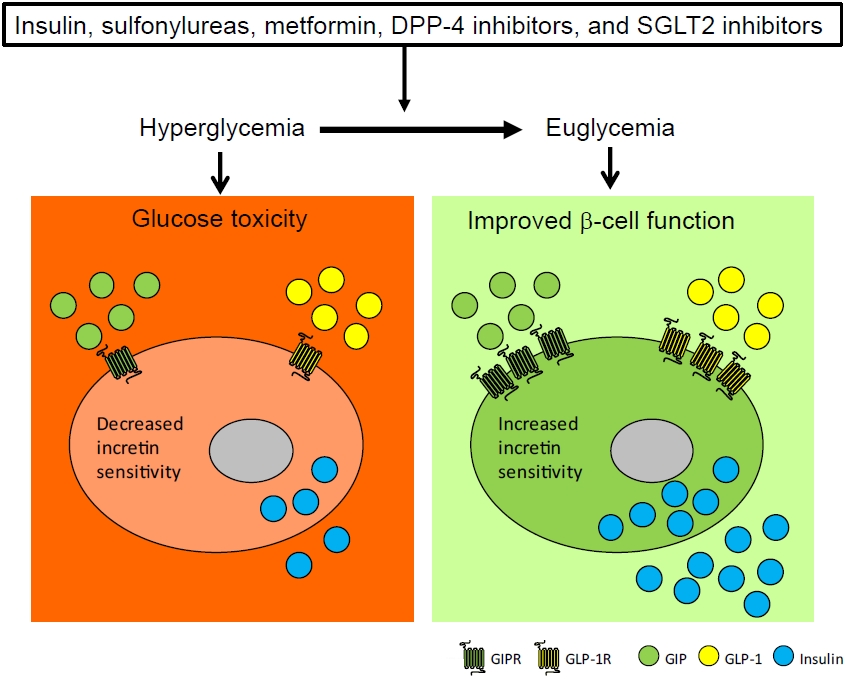
ePub - To maintain normal glucose homeostasis after a meal, it is essential to secrete an adequate amount of insulin from pancreatic β-cells. However, if pancreatic β-cells solely depended on the blood glucose level for insulin secretion, a surge in blood glucose levels would be inevitable after the ingestion of a large amount of carbohydrates. To avoid a deluge of glucose in the bloodstream after a large carbohydrate- rich meal, enteroendocrine cells detect the amount of nutrient absorption from the gut lumen and secrete incretin hormones at scale. Since insulin secretion in response to incretin hormones occurs only in a hyperglycemic milieu, pancreatic β-cells can secrete a “Goldilocks” amount of insulin (i.e., not too much and not too little) to keep the blood glucose level in the normal range. In this regard, pancreatic β-cell sensitivity to glucose and incretin hormones is crucial for maintaining normal glucose homeostasis. In this Namgok lecture 2022, we review the effects of current anti-diabetic medications on pancreatic β-cell sensitivity to glucose and incretin hormones.
-
Citations
Citations to this article as recorded by- Initial Combination Therapy in Type 2 Diabetes
Ji Yoon Kim, Nam Hoon Kim
Endocrinology and Metabolism.2024; 39(1): 23. CrossRef
- Initial Combination Therapy in Type 2 Diabetes

- Clinical Study
- Clinical Characteristics of Subjects with Sulfonylurea-Dependent Type 2 Diabetes
- Se Hee Min, Soo Heon Kwak, Young Min Cho, Kyong Soo Park, Hye Seung Jung
- Endocrinol Metab. 2015;30(4):509-513. Published online December 31, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.4.509
- 4,493 View
- 69 Download
- 3 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Even though several oral anti-diabetic drugs (OAD) with various modes of action are replacing sulfonylurea (SU), some patients seem to be dependent on SU for adequate glycemic control. Therefore, we evaluated the clinical characteristics of such patients.
Methods We selected the patients with type 2 diabetes who met following criteria from 2009 to 2014 at Seoul National University Hospital: glycated hemoglobin (HbA1c) was maintained below 7.5% for at least 6 months under small dose of SU (glimepiride ≤2 mg/day or equivalent dose); after discontinuation of SU, HbA1c increased ≥1.2% within 3 months or ≥1.5% within 6 months; and after resuming SU, HbA1c reduction was ≥0.8% or reduction of fasting plasma glucose was ≥40 mg/dL within 3 months. Patients with impaired hepatic or renal function, and steroid users were excluded.
Results Nineteen subjects were enrolled: after averaged 4.8±1.5 months of SU-free period, HbA1c increased from 6.7%±0.4% to 8.8%±0.8% even though adding other OAD such as gliptins. However, HbA1c decreased to 7.4%±0.7% after resuming SU within 2.4±0.8 months. There was no sexual predominance. Despite their old age (67±11 years) and long duration of diabetes (18±10 years), fasting C-peptide was relatively well-reserved (3.9±2.6 ng/mL), and nephropathy was not observed (albumin-creatinine ratio 21.2±16.6 mg/g and estimated glomerular filtration rate 75.8±18.0 mL/min/1.73 m2). Strong family history was also noted (73.7%).
Conclusion Despite hypoglycemia risk of SU, it seemed indispensable for a subset of patients with regard to insulin secretion. Genetic influences would be evaluated.
-
Citations
Citations to this article as recorded by- Incident Hepatocellular Carcinoma Risk in Patients Treated with a Sulfonylurea: A Nationwide, Nested, Case-Control Study
Ji-Yeon Lee, Suk-Yong Jang, Chung Mo Nam, Eun Seok Kang
Scientific Reports.2019;[Epub] CrossRef - A genetic variant in GLP1R is associated with response to DPP-4 inhibitors in patients with type 2 diabetes
Eugene Han, Hye Sun Park, Obin Kwon, Eun Yeong Choe, Hye Jin Wang, Yong-ho Lee, Sang-Hak Lee, Chul Hoon Kim, Lee-Kyung Kim, Soo Heon Kwak, Kyong Soo Park, Chul Sik Kim, Eun Seok Kang
Medicine.2016; 95(44): e5155. CrossRef
- Incident Hepatocellular Carcinoma Risk in Patients Treated with a Sulfonylurea: A Nationwide, Nested, Case-Control Study


 KES
KES

 First
First Prev
Prev



